
Among the many problems presented by using fossil fuels such as petroleum, one of the more pressing issues is their limited and rapidly decreasing supply. Unfortunately, it would take thousands of years of organic matter decomposing and compressing under layers of the Earth to replenish the supply in the manner in which it was first made, and that’s an unlikely business.
But now it’s been reported that a professor from Kyoto University and his team have found a way to create petroleum efficiently and cheaply. Their method uses no energy-consuming high pressures or temperatures and only requires water, petroleum, and carbon dioxide. As a result, it can be done so cheaply that KTV reported 100 yen (US$0.83) of oil can be synthesized using only 3 yen ($0.02) worth of electricity.
It all seems to good to be true, and in fact it may not be true. With published peer-reviewed studies, mysterious television appearances, and lack of mainstream media coverage. We honestly can’t figure out is this amazing breakthrough or not. And neither can anyone else as science enthusiasts take to Twitter to find answers.
■ The Process
Attempts at synthesizing oil have been made before but the biggest challenge was the high levels of energy required to make the hydrocarbons (chains of hydrogen and carbon) which we need for combustion. For example, one company in Iceland uses the extreme temperatures and pressures of volcanoes to create these hydrocarbons from CO2. Although effective it only works in very specific environmental and economic conditions.
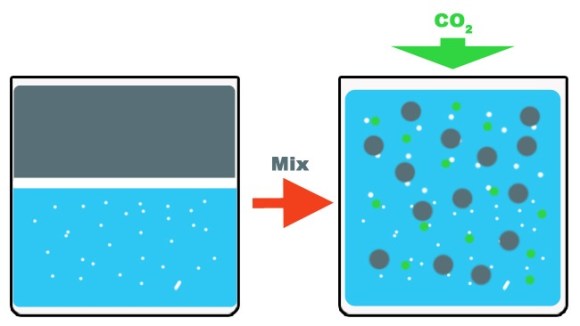
However, Professor Tadayuki Imanaka’s technique can be done anywhere with very little energy and just a few pieces of specialized equipment. The first step involves creating an amount of activated water. This is made with nanobubbles (very, very small bubbles) of oxygen in electrolysed water under UV light along with a catalyst.
Then petroleum is mixed in with the activated water. As the saying goes, oil and water don’t mix so it needs some substantial shaking to get an emulsion. While the oil and water are blending together a substance containing CO2 is added to the mix.
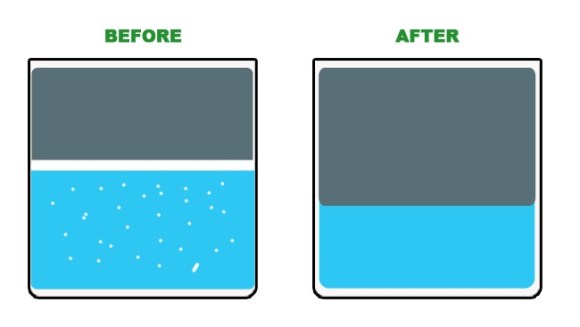
After when the mixture settles and separates again the amount of water is decreased but the amount of petroleum is increased. Imanaka says that the amount of increase depends on the type of oil used such as kerosene or light oil, but ranges from 5 to 10 percent.
■ The potential
Imanaka is confident that this method is effective and hopes a system of mass production can be developed as early as next year. After that his synthetic oil can be made for use in the market in large and cheap quantities.
He also claims this oil will be cleaner burning since it doesn’t release certain greenhouse gases that contain sulfur and nitrogen like natural crude oil does. Furthermore, synthesizing Imanaka’s oil would require collecting and using carbon dioxide which could help in reducing its impact on the environment as well.
This form of petroleum does have some obvious drawbacks. Firstly it requires water which also isn’t a limitless resource. And it is still oil which does result in pollution when burned for energy.
On the other hand, if successful, Imanaka’s oil could completely change the landscape of oil production. Controversial methods of extraction like oil sands or fracking would no longer be as economically attractive. Also, since this oil could be produced anywhere the risk of spills in the ocean from drilling or transporting could be reduced.
It would also level the playing field economically, but not too drastically. Every country would be able to produce their own oil with much less reliance on foreign sources. However, since petroleum is required to make more of this synthetic oil, those nations with existing stocks would still have an advantage in its production.
It certainly has the potential to be a game-changer but whether or not Imanaka’s oil is truly as efficient as he claims remains to be seen. In fact, outside of a few scant reports very little of this potentially ground breaking procedure can be seen at all.
■ The rug pulled out?
Online rumors are swirling over whether this announcement is real at all. There seem to be compelling facts on both sides of the debate as to whether this is real or some kind of mistake.
Starting with the fundamentals, Tadayuki Imanaka is real and works out of the Environmental Biotechnology Lab at Ritsumeikan University in Kyoto. He has worked on projects to create petroleum using bacteria as well as experiments using nanobubbles. However, nowhere on the Ritsumeikan website does it mention this particular research, nor is there any kind of press release from the school to be found.
▼ “I can’t figure out if this article is accurate or not. Can anyone post a link to a press release or article?”
[tweet https://twitter.com/Tamiful07/status/649903067115057153 align=center]
And yet in the original reports there is a photo of what appears to be a televised press conference with Imanaka speaking in front of Kyoto University emblems while the text “Using CO2 gas and water to make petroleum?” is superimposed on the TV screen.
[tweet https://twitter.com/alfalfafafa/status/650108510772989952 align=center]To make matters weirder, the original reports from September 18 that have featured this photo have since been taken down. They can still be access through Google’s cache function, however. Also, even though this story came out way back in mid-September, there has been no mention of it in any of Japan’s major news outlets such as Asahi, Yomiuri, or NHK.
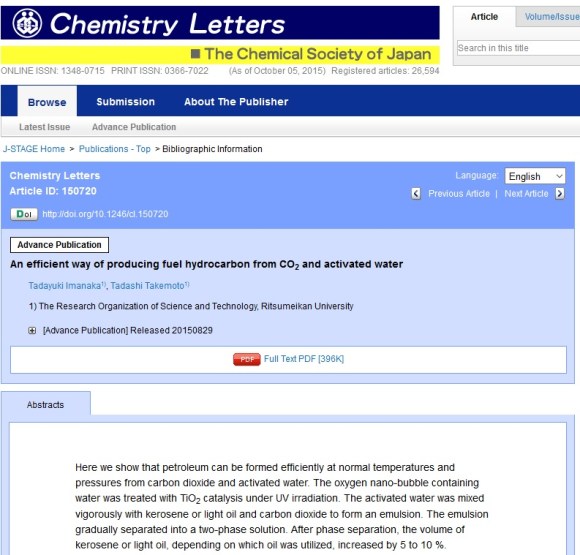
Generally, at this point I’d assume that this was some sort of mistake or hoax. However, the actual study by Imanaka exists in Chemistry Letters, a peer-reviewed journal by the Chemical Society of Japan.
So what is going on here? It’s difficult to say for sure, but based on what we know I’d guess that some news agencies may have jumped the gun a little on how far along Imanaka’s research is in this area. Perhaps more work is needed to determine the stability and performance of this synthetic petroleum. The other news agencies are likely waiting for something more concrete to print, especially since many may still be licking their wounds of embarrassment after trumpeting the STAP cell breakthrough which turned out to be one of the worst scandals of 2014…and there were some doozies that year.
Source: KTV (Cached), ABC News (Cached), Togetter, Itai News , Ritsumeikan University, J-Stage Chemistry Letters – An efficient way of producing fuel hydrocarbon from CO2 and activated water
Top image: Wikipedia/Trevor MacInnis (edited by RocketNews24)

 Japan has only one airport named after a samurai, so let’s check out Kochi Ryoma【Photos】
Japan has only one airport named after a samurai, so let’s check out Kochi Ryoma【Photos】 Japanese Prime Minister once criticized deploying military to fight Godzilla
Japanese Prime Minister once criticized deploying military to fight Godzilla How to make epic pancakes with your Japanese rice cooker
How to make epic pancakes with your Japanese rice cooker Extreme Budget Travel! Can you do a trip to Manila with 50,000 yen (US$333)? – Part 2
Extreme Budget Travel! Can you do a trip to Manila with 50,000 yen (US$333)? – Part 2 Who is this mysterious large man who’s suddenly showing up on giant signs in Japan?
Who is this mysterious large man who’s suddenly showing up on giant signs in Japan? Japan has only one airport named after a samurai, so let’s check out Kochi Ryoma【Photos】
Japan has only one airport named after a samurai, so let’s check out Kochi Ryoma【Photos】 Japanese Prime Minister once criticized deploying military to fight Godzilla
Japanese Prime Minister once criticized deploying military to fight Godzilla How to make epic pancakes with your Japanese rice cooker
How to make epic pancakes with your Japanese rice cooker Extreme Budget Travel! Can you do a trip to Manila with 50,000 yen (US$333)? – Part 2
Extreme Budget Travel! Can you do a trip to Manila with 50,000 yen (US$333)? – Part 2 Who is this mysterious large man who’s suddenly showing up on giant signs in Japan?
Who is this mysterious large man who’s suddenly showing up on giant signs in Japan? Infographic shows how working culture differs across the globe
Infographic shows how working culture differs across the globe Pokémon Smart Bath Mats are amazingly cute, but there’s more to them than meets the eye【Photos】
Pokémon Smart Bath Mats are amazingly cute, but there’s more to them than meets the eye【Photos】 Japanese airlines offer discounted fares to international tourists
Japanese airlines offer discounted fares to international tourists Shimane has a secret hot spring town that feels like stepping into an old Japanese film
Shimane has a secret hot spring town that feels like stepping into an old Japanese film What’s up with the secret basement at this Japanese train station?
What’s up with the secret basement at this Japanese train station? The 10 most annoying things foreign tourists do on Japanese trains, according to locals
The 10 most annoying things foreign tourists do on Japanese trains, according to locals Starbucks Japan releases new sakura goods and drinkware for cherry blossom season 2026
Starbucks Japan releases new sakura goods and drinkware for cherry blossom season 2026 Is Sapporio’s Snow Festival awesome enough to be worth visiting even if you hate the snow? [Pics]
Is Sapporio’s Snow Festival awesome enough to be worth visiting even if you hate the snow? [Pics] Japan has trams that say “sorry” while they ride around town…but why?
Japan has trams that say “sorry” while they ride around town…but why? Tokyo Skytree turns pink for the cherry blossom season
Tokyo Skytree turns pink for the cherry blossom season Highest Starbucks in Japan set to open this spring in the Tokyo sky
Highest Starbucks in Japan set to open this spring in the Tokyo sky Japan’s new “Cunte” contact lenses aren’t pronounced like you’re probably thinking they are
Japan’s new “Cunte” contact lenses aren’t pronounced like you’re probably thinking they are Shibuya Station’s Hachiko Gate and Yamanote Line stairway locations change next month
Shibuya Station’s Hachiko Gate and Yamanote Line stairway locations change next month Yakuzen ramen restaurant in Tokyo is very different to a yakuza ramen restaurant
Yakuzen ramen restaurant in Tokyo is very different to a yakuza ramen restaurant Starbucks Japan adds new sakura Frappuccino and cherry blossom drinks to the menu
Starbucks Japan adds new sakura Frappuccino and cherry blossom drinks to the menu Japan’s newest Shinkansen has no seats…or passengers [Video]
Japan’s newest Shinkansen has no seats…or passengers [Video] Foreigners accounting for over 80 percent of off-course skiers needing rescue in Japan’s Hokkaido
Foreigners accounting for over 80 percent of off-course skiers needing rescue in Japan’s Hokkaido Super-salty pizza sends six kids to the hospital in Japan, linguistics blamed
Super-salty pizza sends six kids to the hospital in Japan, linguistics blamed Starbucks Japan unveils new sakura Frappuccino for cherry blossom season 2026
Starbucks Japan unveils new sakura Frappuccino for cherry blossom season 2026 Foreign tourists in Japan will get free Shinkansen tickets to promote regional tourism
Foreign tourists in Japan will get free Shinkansen tickets to promote regional tourism Take a trip to Japan’s Dododo Land, the most irritating place on Earth
Take a trip to Japan’s Dododo Land, the most irritating place on Earth Naruto and Converse team up for new line of shinobi sneakers[Photos]
Naruto and Converse team up for new line of shinobi sneakers[Photos] Is China’s don’t-go-to-Japan warning affecting the lines at a popular Tokyo gyukatsu restaurant?
Is China’s don’t-go-to-Japan warning affecting the lines at a popular Tokyo gyukatsu restaurant? Survey asks foreign tourists what bothered them in Japan, more than half gave same answer
Survey asks foreign tourists what bothered them in Japan, more than half gave same answer Japan’s human washing machines will go on sale to general public, demos to be held in Tokyo
Japan’s human washing machines will go on sale to general public, demos to be held in Tokyo Starbucks Japan releases new drinkware and goods for Valentine’s Day
Starbucks Japan releases new drinkware and goods for Valentine’s Day We deeply regret going into this tunnel on our walk in the mountains of Japan
We deeply regret going into this tunnel on our walk in the mountains of Japan Studio Ghibli releases Kodama forest spirits from Princess Mononoke to light up your home
Studio Ghibli releases Kodama forest spirits from Princess Mononoke to light up your home Major Japanese hotel chain says reservations via overseas booking sites may not be valid
Major Japanese hotel chain says reservations via overseas booking sites may not be valid Put sesame oil in your coffee? Japanese maker says it’s the best way to start your day【Taste test】
Put sesame oil in your coffee? Japanese maker says it’s the best way to start your day【Taste test】 No more using real katana for tourism activities, Japan’s National Police Agency says
No more using real katana for tourism activities, Japan’s National Police Agency says Infographic shows how working culture differs across the globe
Infographic shows how working culture differs across the globe Pokémon Smart Bath Mats are amazingly cute, but there’s more to them than meets the eye【Photos】
Pokémon Smart Bath Mats are amazingly cute, but there’s more to them than meets the eye【Photos】 Japanese airlines offer discounted fares to international tourists
Japanese airlines offer discounted fares to international tourists Shimane has a secret hot spring town that feels like stepping into an old Japanese film
Shimane has a secret hot spring town that feels like stepping into an old Japanese film What’s up with the secret basement at this Japanese train station?
What’s up with the secret basement at this Japanese train station? Survey finds that one in five high schoolers don’t know who music legend Masaharu Fukuyama is
Survey finds that one in five high schoolers don’t know who music legend Masaharu Fukuyama is World Record? Japanese trainer holds gym for 1,422 days and counting in Pokémon GO
World Record? Japanese trainer holds gym for 1,422 days and counting in Pokémon GO Japan has trams that say “sorry” while they ride around town…but why?
Japan has trams that say “sorry” while they ride around town…but why? Japan’s new “Cunte” contact lenses aren’t pronounced like you’re probably thinking they are
Japan’s new “Cunte” contact lenses aren’t pronounced like you’re probably thinking they are Japan Extreme Budget Travel! A trip from Tokyo to Izumo for just 30,000 yen [Part 1]
Japan Extreme Budget Travel! A trip from Tokyo to Izumo for just 30,000 yen [Part 1] Family Mart’s Shibuya Cat Street shop hosts first-ever rescue cat photo exhibition for Cat Day
Family Mart’s Shibuya Cat Street shop hosts first-ever rescue cat photo exhibition for Cat Day Beautiful cans of cake become a viral hit in Japan
Beautiful cans of cake become a viral hit in Japan Tokyo’s best ramen breakfast? Restaurant two minutes from Tokyo Station is a strong contender
Tokyo’s best ramen breakfast? Restaurant two minutes from Tokyo Station is a strong contender